MedFriendly®


Anion
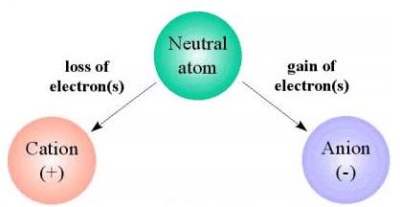
An anion is an ion with a negative electric
charge. An ion is an atom or a group of atoms
that have an electric charge by gaining or losing
one or more electrons. An atom is the smallest
part of a substance that can exist alone or in
combination with something else. An electron is
a negatively charged particle that is smaller than
an atom. Since an electron has a negative
charge, if an atom gains too many electrons it is
considered negative (because there will be
more negative charges than positive charges).
Anions have a negative charge.
Cations have a positive charge.
FEATURED BOOK: Anion Coordination Chemistry
Anions travel towards a positive pole known as an anode. A pole is one of two points
that are at the extremes (for example, the positive and negative poles). Examples of
anions are phosphate (a type of salt) and bicarbonate. Bicarbonate is a substance in the
blood that prevents it from becoming too acidic or too alkaline (non-acidic).
See the entry for ions to learn how anions are important to the body and how abnormal
levels of anions can cause problems. Compare anions to cations, which are ions with
positive electrical charges. Anion is sometimes abbreviated as A-. Anionic means
pertaining to an anion. The word "anion" comes from the Greek word “ano” meaning “up”
and the Greek word “ion” meaning “going.” Put the two words together and you get “going
up” which is a reference to gaining electrons.
"Where Medical Information is Easy to Understand"™