MedFriendly®


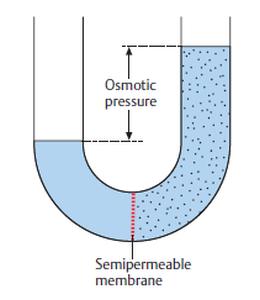
Osmotic Pressure
BASIC TERMS NEEDED TO UNDERSTAND OSMOTIC
PRESSURE?
To understand what osmotic pressure is, it is first necessary
to understand a few other terms. If you already know the
following terms, feel free to skip to the next section.
SOLVENT: A usually liquid substance (such as water) that is
capable of dissolving or dispersing one or more substances.
SOLUTE: A substance (such as sugar) that dissolves in a
solvent. So if you poured sugar into a cup of water, the sugar
would be the solute and the water would be the solvent.
Water passes from the solution
with less solutes to the solution
with more solutes.
FEATURED BOOK: Chemistry for Dummies
SEMIPERMEABLE MEMBRANE: A thin layer of tissue that allows some substances in,
but not all. For example, the semipermeable membrane may allow a smaller substance in
but not a larger one.
Alternatively, it may only allow the solvent (such as water) to pass through, but not allow
any solutes to pass through. Semipermeable membranes are widespread in the body and
surround all cells.
"Where Medical Information is Easy to Understand"™
WHAT IS OSMOTIC PRESSURE?
1. The pressure that needs to be applied to a solution to stop the
movement of a solvent into it, when the solution and solvent (such as
water) are separated by a semipermeable membrane that only allows
the solvent to pass through. In other words, although the
semipermeable membrane would normally allow the solvent to pass
through it, osmotic pressure prevents the solvent from passing
through. Osmotic pressure can prevent osmosis, which is the
movement of a solvent (such as water) through a semipermeable
membrane, from a solution that has a lower concentration of solutes
to one that has a higher concentration of solutes.
2. The force in which a solution attracts a solvent (such as water) through a semipermeable membrane.
This definition of osmotic pressure is less commonly used than the first.
WHAT DOES OSMOTIC PRESSURE DEPEND ON?
Osmotic pressure depends on the amount of solute that is present in the solvent (such as water). It also
depends on the temperature of the solution.
WHAT IS THE ORIGIN OF THE TERM "OSMOTIC PRESSURE"?
Osmotic pressure comes from the Greek word "osmos" meaning "impulse," the Greek word "ikos" meaning
"pertaining to," and the Latin word "premere" meaning "to press." Put the words together and you have
"pertaining to impulse to press."