

MedFriendly®


Atom
An atom is the smallest part of a substance that
can exist and still possess all of the properties
that are characteristic of the substance. An atom
is also the smallest part of a substance that can
exist alone or in combination with something else.
Atoms cannot be seen, even with high-powered
equipment. The word "atomic" means pertaining
to an atom.
Diagram of an atom.
FEATURED BOOK: Chemistry: Atoms First
CAN ATOMS BE DIVIDED?
Yes. Although the word atom comes from the Greek word "atomos" meaning "indivisible,"
we know today that atoms can be divided. This knowledge came about with the
discovery of radioactivity, which is the property of a nucleus (the center of an atom) to
break up by itself and send out a form of energy known as radiation.
Radioactivity proved that particles existed that were smaller than atoms (for example, the
particles that make up the nucleus).
The nucleus, and the particles that make up the nucleus are described in more detail
below.
"Where Medical Information is Easy to Understand"™
WHAT IS THE INSIDE OF AN ATOM MADE OF?
The inside of an atom is complex. The outer area
of an atom consists of a lot of empty space.
However, in the center of an atom there is a very
very tiny structure known as the nucleus. Although
it is very small, the nucleus makes up almost all of
the mass (amount of matter in an object) of an
atom. The nucleus is so small that its radius is
about 1/10,0000th that of an atom. Radius means
the length of a straight line extending from the
center of a circular structure to the edge of that
structure.
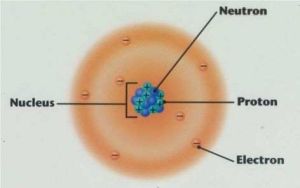

WHAT IS THE NUCLEUS MADE OF?
The nucleus is made up of particles known as protons and neutrons. Together, protons and neutrons are
known as nucleons. Protons are positively charged particles, whereas neutrons have no electric charge.
To the right is a drawing of a nucleus, with the protons and neutrons shown.
WHAT IS GOING ON IN THE SPACE OUTSIDE OF THE NUCLEUS?
Outside of the nucleus, there are areas of space that contain moving electrons (negatively charged
particles). These areas of space are called electron shells. Atoms can have up to seven electron shells.
The electron shell closest to the nucleus is known as the K shell. As the shells move out from the center,
they are known as the L shell, the M shell, the N shell, the O shell, the P shell, and the Q shell, in that
order.
Each electron shell can hold a certain number of electrons. For example, the K shell can hold 2 electrons,
the L shell can hold 4 electrons, the M shell can hold 8 electrons, the N shell can hold 18 electrons, and
the O shell can hold 32 electrons. The farther away an electron shell is from the nucleus, the more energy
its electrons have. The outer shell is the last shell with electrons in it. This can be any of the shells (K
through Q), it just depends on how many shells are in the atom. If the outer shell has 8 electrons or is full
of electrons, the atom is considered very stable (not changing).
CAN THE POSITIONS OF THE ELECTRONS IN THEIR ELECTRON SHELL BE DETERMINED AT A
GIVEN TIME?
No. The positions of the electrons in their electron shells cannot be exactly determined at any given time.
However, each electron shell is made up of areas known as orbitals (also known as probability clouds).
Orbitals are areas in which one or two electrons are likely to be found at a given time.
NOW THAT I READ ALL THAT, COMPARE PROTONS, NEUTRONS, AND ELECTRONS.
Protons have a positive electric charge, electrons have a negative electric charge, and neutrons have no
electric charge. Since protons and electrons have opposite electric charges, the charges cancel each
other out. By canceling each other out, atoms are electrically neutral (meaning they have no electric
charge).
Another comparison of protons, neutrons, and electrons, relates to size. As was mentioned earlier, an
electron is very small. Electrons are much smaller than protons and neutrons. Specifically, the mass of a
neutron is about 1,840 times that of an electron. The mass of a proton is about 1,836 times that on an
electron.
DO ALL ATOMS HAVE THE SAME NUMBER OF PROTONS AND NEUTRONS?
No. The number of protons and neutrons that atoms have differ depending on what element they come
from. Elements (such as oxygen or hydrogen) are identified by the number of protons that they have. The
number of protons in a nucleus is known as the atomic number (abbreviated as Z). The number of protons
is equal to the number of electrons. The number of neutrons in atoms of the same element can differ. The
number of neutrons in a nucleus is known as the neutron number (abbreviated as N). The number of
protons and neutrons in a nucleus is known as the mass number (abbreviated as A).
CAN THERE BE DIFFERENT FORMS OF THE SAME ELEMENT?
Yes. Different forms of the same element (such as oxygen or hydrogen) are known as isotopes. These
elements have the same number of protons but different numbers of neutrons as compared to the natural
form of the element. Every element has an isotope. Isotopes made by man are known as radioisotopes.
IS THERE ANYTHING SMALLER THAN PROTONS OR NEUTRONS?
Yes. Protons are made up of even smaller substances known as quarks. Quarks are only found in groups.
There are six types of quarks, each of which have their own special names: up quarks, down quarks, top
quarks, bottom quarks, charm quarks, and strange quarks. Protons are made of two up quarks and one
down quark. Neutrons are made of one up quark and two down quarks. When quarks come together to
form particles, these particles are known as hadrons. Protons and neutrons are types of hadrons.
As was mentioned earlier, electrons are smaller than protons and neutrons, but they do not make up the
protons and neutrons like the quarks do. Electrons belong to a class of particles known as leptons. There
are five other types of leptons besides electrons. They are known as the muon, the tau, and three types of
neutrinos. The muon and the tau both have negative electric charges. The three types of neutrinos have
no charge, and little, if any mass.
IS THERE ANYTHING SMALLER THAN QUARKS OR ELECTRONS?
Scientists now believe that quarks and electrons cannot be divided and that they are the fundamental
substances that all matter is made of. However, scientists are still investigating whether this is true. It is
possible that electrons and quarks are made up of smaller substances that have yet to be discovered.
Remember that at one point in time it was believed that atoms could not be divided.
WHAT IS ATOMIC WEIGHT?
Atomic weight (also known as atomic mass or relative atomic mass) is the amount of matter in an atom. In
other words, it is the amount of substance in an atom. Do not take the word "weight" literally, since it is
impossible to weigh an object as small as an atom. This is why the word "mass" is the best term to use
since it means the amount of substance in an atom.
Atomic weight or atomic mass is not measured in pounds, of course, so what is the unit of measurement
for an atomic weight/atomic mass? The surprising answer is that they are unitless! That is, we do not
know how to find the mass of an individual atom by itself. What we can do is find the mass of a whole
bunch of atoms by comparing them to the mass of another bunch of atoms. This is where the term relative
atomic mass comes in, because the mass of atoms are measured by comparing one bunch of atoms
relative to (in relation to) another bunch of atoms, of the same sizes. This is easy to do if the same
numbers of atoms are in each bunch.
To make this easier to understand, let's pretend that paperclips the same thing as atoms (after all,
paperclips are pretty small). Let's also assume that we have two types of paperclips: white paperclips and
red paperclips. The two types of paperclips are in two piles. Let's also assume that we have the same
amount of paperclips in each pile and that the paperclips are the same sizes.
If we put one paperclip on a scale, it would be very hard to weigh it because the scale would probably not
be sensitive enough. However, if we put a whole bunch of paperclips on the scale, then we would be able
to weigh them. So let's say we put a whole bunch of white paperclips on one scale and a whole bunch or
red paperclips on another scale.
Now let's say that the white paperclips weighed 2 grams and that the red paperclips weighed 10 grams. A
gram is a very small unit of weight (you would need 453.359237 grams just to get one pound!) Now we
can compare the weight of the red paperclips to the weight of the white paperclips. To do this, we just
divide 10 (the weight of the red paperclips) by two (the weight of the white paperclips), and we get 5. We
can create a name for the units of weight for the paperclips (let's call them relative paperclip mass units).
So now we can say that the red paperclips have 5 relative paperclip mass units. The relative paperclip
mass units of the white paperclips would be 1. This is because two divided by two equals one.
Can we do the same type of equation as the one above with two different types atoms? Yes. All you need
to do is have some way of knowing that you have the same amount of atoms for each type of atom. But
how can we do this if we can't even see atoms? It requires believing in a hypothesis (an uncertain
assumption) put forth by a scientist named Lorenzo Romano Amedeo Carlo Avogadro di Quareqa e di
Carreto. Let's just call him Avogadro. The hypothesis is known as Avogadro's hypothesis and it says that
the same volumes (amounts of space occupied by something) of gases at the same temperature and
pressure contain the same numbers of molecules. Molecules are the smallest naturally occurring particles
of a substances and are made of any number of atoms, from one to thousands.
Keeping Avogadro's hypothesis in mind, let's say we have two containers of gas: one container has
hydrogen in it while the other container has oxygen in it. The hydrogen and oxygen are both at room
temperature and have the same pressures. If we believe in Avogadro's hypothesis, this means that the
number of molecules in each container is the same.
We have techniques to measure the weight of gases, so let's say that the hydrogen weighs .23 grams and
the oxygen weighs 3.68 grams. Now we can compare the weight of the oxygen gas to the weight of the
hydrogen gas. To do this, we just divide 3.82 (the weight of the oxygen gas) by .23 (the weight of the
hydrogen gas), and we get 16. We can create a name for the unit of weight for the gasses (let's call it
relative gas mass unit). So now we can say that the oxygen gas has 16 relative gas mass units.
We can also say that an oxygen atom is 16 times heavier than a hydrogen atom because atoms make up
the gas. In other words, since the oxygen gas is 16 times heavier than the hydrogen gas, the oxygen
atoms must be 16 times heavier than the oxygen atoms too. The official name for the units of mass for an
atom is called unified atomic mass units (abbreviated as u). The unified atomic mass units for hydrogen
would be 1. This is because .23 divided by .23 equals one.
If you look in a textbook, you will see that the relative mass of hydrogen equals 1.00794 u. Why not just
1? There are two main reasons:
1. As was mentioned earlier, atoms have isotopes (different forms of the same element due to different
numbers of neutrons). The atomic number that you see in textbooks for an element is the average of the
atomic masses for the natural element and its isotopes.
2. All elements are compared to an element known as carbon-12. It is called carbon-12 because it has 6
protons and 6 neutrons. The atomic mass of carbon-12 is equal to 12. Since all elements have their
weights divided by 12, it worked out that no other atomic masses came out to a whole number.
WHAT FIELDS OF SCIENCE DEALS WITH THE STUDY OF ATOMS?
Atoms are typically studied in chemistry (the science of elements, combinations of elements, the structure
of elements, and the interaction of different types of matter) and physics (the study of matter and energy,
especially as they relate to motion and force).
WHAT IS THE ORIGIN OF THE TERM, ATOM?
Atom comes from the Greek word "atomos" meaning "indivisible." The ancient Greeks believed that all
matter was made up of tiny particles called atoms.














