MedFriendly®


Osmosis
BASIC TERMS NEEDED TO UNDERSTAND
OSMOSIS:
To understand what osmosis is, it is first necessary to
understand a few other terms. If you already know the
following terms, feel free to skip to the next section.
SOLVENT: A usually liquid substance (such as water)
that is capable of dissolving or dispersing one or more
substances.
SOLUTE: A substance (such as sugar) that dissolves
in a solvent. So if you poured sugar into a cup of water,
the sugar would be the solute and the water would be
the solvent.
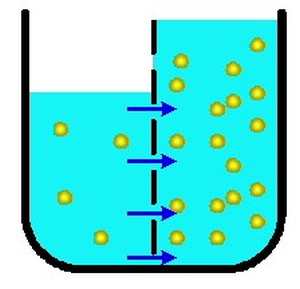
In osmosis, water moves from the
area of lower concentration to
higher concentration.
FEATURED BOOK: Chemistry for Dummies
SOLUTION: The combination of a solute with a solvent, when the solute has been evenly
dissolved in the solvent. An example would be when sugar (the solute) is evenly dissolved
in a glass of water (the solvent).
CONCENTRATION: A measurement of the amount of a solute compared to the amount of
solvent in a solution. As an example, if a high amount of sugar (the solute) is dissolved in
a small amount of water (the solvent), the concentration would be high. The concentration
would be low if a small amount of sugar was dissolved in a large amount of water.
"Where Medical Information is Easy to Understand"™
SEMIPERMEABLE MEMBRANE: A thin layer of tissue that allows
some substances in, but not all. For example, the semipermeable
membrane may allow a smaller substance in but not a larger one. Or
it may only allow the solvent (such as water) to pass through, but not
allow any solutes to pass through. Semipermeable membranes are
widespread in the body and surround all cells.
WHAT IS OSMOSIS?
Osmosis is the movement of a solvent (such as water) through a
semipermeable membrane, from a solution that has a lower
concentration of solutes to one that has a higher concentration of
solutes.
See the section above for a description of some of these terms. In osmosis, the membrane does not allow
the solute to pass through, but does allow the solvent to pass through. If we stick with the example of
sugar being the solute and water being the solvent, the sugar would not be able to pass through the
membrane, but the water would be able to pass through. After osmosis, the concentrations of the solute
are more equal on both sides of the membrane.
WHAT IS AN EXAMPLE OF OSMOSIS?
To make osmosis easier to understand, here is an example. Let's say that we have a cup of water that is
divided by a membrane. There is an equal amount of water (the solvent) on both sides of the membrane.
On both sides of the membrane, there is also an uneven amount of sugar (the solute) in the water. On the
left side, there are 2 tablespoons of sugar and on the right side there is only one tablespoon of sugar. So
the concentration of sugar is higher on the left side than the right.
In osmosis, the water is going to move through the membrane from the right side of the cup (where there
is less sugar) to the left side of the cup (where there is more sugar). Now there is going to be an unequal
amount of water in the cup, with more water on the left side than the right. This causes the level of sugar
to be equal on both sides of the cup. In sum, whereas we started off with an equal amount of water on
both sides and an unequal amount of sugar, through osmosis we wind up with an unequal amount of water
on both sides and an equal amount of sugar.
WHEN DOES OSMOSIS OCCUR?
Osmosis occurs whenever a semipermeable membrane separates two solutions of different
concentrations.
HOW FAST DOES OSMOSIS TAKE?
The rate in which osmosis occurs depends on the temperature of the solution, the concentration of the
solute, and the electrical charge of the solute. Another factor is the difference between the pressures that
both sides of the solutions exert onto the membrane. See the beginning of this entry for a description of
the terms "solution" and "solute."
WHEN DOES OSMOSIS STOP?
Osmosis stops when the two solutions are equal in concentration. The only exception to this is when the
movement of a solvent (such as water) is prevented by applying pressure to the more concentrated
solution. The pressure required to stop the movement completely is called osmotic pressure.
WHY IS OSMOSIS IMPORTANT?
Osmosis is important because it helps determine how water and other substances are distributed in the
body.
WHAT IS THE ORIGIN OF THE TERM "OSMOSIS"?
Osmosis comes from the Greek word "osmos" meaning "impulse," and the Greek word "osis" meaning
"condition." Put the two words together and you have "impulse condition."