Double click to edit


MedFriendly®
HOW DOES ABACAVIR WORK?
Abacavir works by decreasing the ability of HIV to reproduce, resulting in less HIV in
the blood and decreases the chance of HIV complications (e.g., new infections,
cancer). It is technically classified as a nucleoside analog reverse transcriptase
inhibitor (NRTI) due to how it prevents HIV from reproducing. What happens is that
when abacavir gets into the body it is changed by an enzyme into a substance called
carbovir triphosphate. An enzyme is a type of protein that helps produce chemical
reactions in the body.
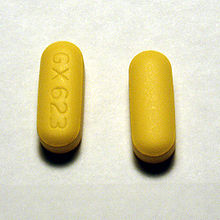
As seen in the above
picture, abacavir tablets
are yellow, scored,
rounded outwards on both
sides, covered with a film,
and are imprinted with “GX
623” on both sides.
Abacavir (abacavir sulfate; trade names = Ziagen; abbreviated ABC) is a
drug used to combat HIV (human immunodeficiency virus) and AIDS
(Acquired Immune Deficiency Syndrome) in combination with other HIV
medications. HIV is a virus that attacks the body’s immune (defense)
system, leading to infections and harmful tumors (tissues that grow more
rapidly than normal). AIDS is a decrease in the effectiveness of the body's
immune (defense) system that is due to HIV infection.
When abacavir is combined with zidovudine (AZT) and lamivudine (3TC),
the combination is known as Trizivir. As noted above, abacavir is not used
alone but in combination with other medications. When abacavir is
combined with lamivudine it is known as Kivexa/Epzicom. Some HIV strains
that are resistant to the effects of AZT or 3TC are generally susceptible to
abacavir whereas HIV strains resistant to the effects of AZT and 3TC are
not as sensitive to abacavir.
Carbovir triphosphate is very similar to a chemical (known as deoxyguanosine triphosphate or dGPT) that
HIV needs to make new DNA (deoxyribonucleic acid) and thus competes with it. DNA is a chain of many
connected genes. Genes contain coded instructions for how proteins should be constructed, such as
those that make up the HIV virus. HIV needs to make new DNA for each virus and it normally does so by
using a specific enzyme known as reverse transcriptase. However, when abacavir works, carbovir
triphosphate competes with the dGBT that is needed by reverse transcriptase and is incorporated into the
viral DNA.
As a result, reverse transcriptase is unable to copy its genetic material to generate new virus material
because carbovir triphosphate interferes with it. For this reason, abacavir is known as a reverse
transcriptase inhibitor. Carbovir triphosphate lacks a chemical group known as 3’-OH which prevents the
formation of a chemical bond needed for the HIV viral chain to continue.
When HIV stops reproducing because the viral chain cannot extend its form, this is known as chain
termination. Abacavir does not kill existing HIV and this cannot cure it, but it can help stop it from
reproducing. Strains of HIV that are resistant to a different class of HIV medications known as protease
inhibitors are not usually resistant to abacavir. It is unknown if abacavir decreases the risk of transmitting
HIV to others.
WHAT IS ABACAVIR HYPERSENSITVITY SYNDROME?
Abacavir is considered a generally well tolerated medication (in 90% of people). The main side effect is
hypersensitivity (known as abacavir hypersensitivity syndrome or AHS), which occurs in about 2 to 9% of
patients during the first six weeks of treatment but most people develop it after 9 to 11 days. Common
breathing/wheezing, fever, malaise (a feeling of being unwell), gastrointestinal tract symptoms (nausea,
vomiting, diarrhea, stomach pain), respiratory symptoms (shortness of breath, cough, sore throat), skin
rash, swelling, weakness, and fatigue/extreme tiredness.
Other signs and symptoms include itching (face, tongue, and throat), break down of muscle tissue,
swelling, abnormal chest x-ray findings, abnormal sensations, and severe dizziness. There can be
swelling or enlargement of the lymph nodes. Lymph nodes are small egg shaped structures in the body
that help fight against infection. Damage to the mucous membranes can occur. A mucous membrane is
one of four major types of thin sheets of tissue that line or covers various parts of the body. Ulcers (open
sores) in the mouth can occur. Conjunctivitis (inflammation of the conjunctiva) can also occur. The
conjunctiva is a layer that covers and protects the inside of the eyelids and the front part of the sclera
(the white part of the eyes). The skin rash caused by abacavir is often flat and red with small bumps that
are close together. The skin rash can also be pale, red, raised, and itchy. However, other types of skin
rashes can occur due to AHS.
Early diagnosis is difficult because the symptoms of AHS can be confused with the symptoms of HIV,
other infections, hypersensitivity to other medications, and immune restoration disease. Immune
restoration disease is when the restored immune system after highly active antiretroviral therapy
(HAART) is pathological and causes disease. In some cases, there can be worsening of an old medical
condition such as an old infection. The body can have an inflammatory response to opportunistic or
slowly developing infections. This reaction can occur at any time.
In rare cases, AHS can be deadly, which is why the medication is immediately and permanently
discontinued if AHS develops. If someone who has AHS takes abacavir again, or any other medication
containing abacavir again, it can cause very low blood pressure or death within hours. Elevated liver
enzymes (alanine aminotransferase and aspartate aminotransferase) can be present in hypersensitivity
reactions to abacavir. When liver enzymes are elevated it is an indication of liver damage. This is why
people with liver disease need to be careful about using abacavir because it can worsen the problem and
lead to liver failure. Kidney failure and breathing failure can occur in some AHS cases. Adult respiratory
distress syndrome can occur, which is a severe, life-threatening reaction in adult humans due to injuries
or acute infections to the lung. Treatment with abacavir is suspended in cases where organ failure occurs
along with lactic acidosis.
AHS can also be associated with a decrease in lymphocytes. A lymphocyte is a type of white blood cell
present in the blood. White blood cells help protect the body against diseases and fight infections.
Increased creatine levels can also occur due to AHS. Creatinine is a waste product of the normal
breakdown of muscle during activity. Increased creatine phosphokinase can occur due to AHS. Creatine
phosphokinase is an enzyme found mainly in the heart, brain, and skeletal muscle.
AHS is related to how abacavir changes the shape and chemistry of part a protein product coming from a
gene variation known as HLA-B*5701 (also known as B57). These changes affect the body’s immune
system and leads to the activation of cytotoxic T-cells (types of white blood cells) that specifically target
abacavir and releases inflammatory substances (TNF-alpha and IFN-gamma) that result in the delayed
hypersensitivity reaction.
Those who are prone to hypersensitivity reactions can be detected with genetic testing, which is readily
available. On 7/24/08, the Food and Drug Administration (FDA) recommended this genetic testing for
people who have never used abacavir in the past or for those re-initiating treatment with the medication.
It is also recommended that people check with their doctor if they miss several doses of abacavir before
restarting it. For those who have a positive genetic test result, there is a 50% chance of developing AHS.
For those with a negative genetic test result, it is very unlikely that the person will develop AHS. One
study, known as the PREDICT-1 study, found that 7.8% (66/847) of patients treated with abacavir without
genetic screening developed clinically suspected AHS compared to 3.4% (27/803) who only used
abacavir if genetic testing showed they did not have B57. The results also lead to estimates that 61% of
patients who tested positive for the B57 gene would develop AHA compared to 4.5% who tested negative
for B57. Screening for B57 reduced clinically suspected AHA by about 60% compared to if no screening
occurred. Another study known as the SHAPE study also supported the use of genetic screening.
Skin patch testing is a detection method for AHS although it is not as accurate as genetic testing,
sometimes not detecting those who at risk to develop AHS. For this reason, skin patch testing is
considered a research tool and not useful for clinical diagnosis of AHS.
The prevalence of individuals who possess B57 varies according to ethnicity as follows: the Yoruba from
Nigeria (0%), African-Americans (1%), Chinese Americans (1.2%), Hispanic Americans (3%), the Luhya
from Kenya (13.6%), people of European ancestry (3.4 to 5.8%), the Masai from Kenya (13.6%), and
Indian Americans (17.6%). When this gene form is detected, the FDA recommends using another HIV
medication instead of abacavir. There are rare exceptions to this rule of thumb when the benefits clearly
outweigh the risks.
Steven’s-Johnson syndrome and toxic epidermal necrolysis has been suspected in some people using
abacavir in combination with other medications known to be associated with these conditions. Stevens-
Johnson syndrome is a rare but serious condition in which the skin and at least two surfaces of the
mucous membranes (or the mucous membranes only) are damaged by a severe reaction to infection or
medication. A mucous membrane is one of four major types of thin sheets of tissue that line or covers
various parts of the body. TEN is a rare and sometimes life threatening disorder of the skin caused by a
fault in the immune system. There have also been reports of erythema multiforme with abacavir use.
Erythema multiforme is an acute (sudden), self-limited, and sometimes recurring skin condition that is a
hypersensitivity reaction associated with certain medications, infections, and other triggers.
Patients with AHS need to stop using abacavir immediately. When this is done, AHS is usually
reversible.
WHAT ARE OTHER SIDE EFFECTS OF ABACAVIR?
Many patients use abacavir without serious side effects. However, other side effects of abacavir include
anxiety, depression (or worsening of pre-existing depression), diarrhea, fatigue, chills, headache, loss of
appetite, muscle pain, rash, sleep difficulty (falling/staying asleep, strange dreams), triglyceride (fat cell)
level increases in the blood, upper respiratory infection, vomiting, high fat cells (triglycerides) in the
blood, redistribution or accumulation of body fat, vision changes, increased sensitivity to light, and
urinating less or not at all. The most common side effects of abacavir in adults are bad dreams, sleep
problems, nausea, headache, tiredness and vomiting. The most common side effects of abacavir in
children are fever, chills, nausea, vomiting, rash, and infections of the ear, nose, and throat.
Redistribution or accumulation of body fat can cause obesity, fat accumulation at the bottom/back of the
neck, upper shoulders, stomach, face, and breasts, and a wasting appearance of the face, arm, leg, and
buttocks. Accumulation of body fat can be reduced with exercise.
One study indicated a nearly 90% increased risk in heart attack in people who use abacavir. On 3/1/11,
the Food and Drug Administration (FDA) announced a safety review of abacavir and a possible risk of
increased heart attack with the medication. However, the FDA ultimately concluded that while there was
conflicting data on the topic that a review of 26 randomized control trials showed no significant increase
in heart attack between those who used abacavir versus those who did not.
Very rarely, abacavir can cause lactic acidosis (especially in women), in which the high level of acid is
due a buildup of a substance called lactic acid. Lactic acid buildup is what causes the burning feeling in
your muscles when lifting weights for many repetitions. Pancreatitis (inflammation of the pancreas) is
another serious side effect of abacavir. The pancreas is a long organ in the back of the belly that makes
insulin (a substance in the body which helps absorb glucose, a type of sugar). Children using abacavir
have been found to have mild elevations in blood glucose levels compared to adults. Increased GGTP
(gamma-glutamyl transpeptidase) levels have also been found in some people who use abacavir. GGTP
is a type of enzyme that plays a role in metabolism. A severe increase in liver size with an accumulation
of fat can occur with abacavir use, especially in women.
If patients taking abacavir experience blisters, chills, difficulty swallowing/breathing, hives, itching, and
peeling skin it is important to call the doctor immediately as these are considered serious and important.
Side effect frequency of using abacavir once a day (600 mg) is generally similar to using 300 mg of
abacavir twice a day. Hypersensitivity reactions have been shown to occur in 9% of people who use
abacavir once a day (600 mg) compared to 7% of people who use 300 mg of abacavir twice a day.
However, those who use abacavir once a day (600 mg) have been shown to experience significantly
higher rates of severe hypersensitivity reactions compared to people who use abacavir 300 mg twice a
day (5% vs 2%). Those who use abacavir once a day (600 mg) have been shown to experience
significantly higher rates of severe diarrhea compared to people who use abacavir 300 mg twice a day
(2% vs 0%). Another study showed that those who use abacavir once a day (600 mg) experience
significantly higher rates of hypotension with a severe hypersensitivity reaction compared to people who
use abacavir 300 mg twice a day (11% vs 0%).
WHAT FORMS ARE ABACAVIR AVAILABLE IN?
Abacavir is available in tablet and liquid form.
DOES ABACAVIR INTERACT WITH OTHER MEDICATIONS?
Yes. Your doctor should know if someone using abacavir is using other medications to treat HIV or
methadone (Dolophine; Methadose). Methadone is a pain medication that is sometimes used to treat
people who are addicted to heroin, morphine, or other pain reducing drugs. While methadone does not
have a clinically significant effect on abacavir, a study showed that 11 people using twice the daily
recommended dose of abacavir (600 mg twice a day) had increased clearance of methadone from their
body. Thus, such individuals may need an increased methadone dose although this would be in the
minority of cases. Abacavir should not be taken with other medications that contain abacavir.
HOW ARE MISSED DOSES HANDLED?
Doctors advise patients not to double up on a missed dose and to take a missed dose as soon as it is
remembered unless it is close in time to taking the next dose. If it is almost time to take the next dose,
doctors advise skipping the missed dose and to continue the regular dosing. If several doses of
abacavir are missed, doctors advise notifying them before restarting the medication to prevent a
dangerous and sometimes fatal allergic reaction.
WHAT ARE SIGNS OF AN ABACAVIR OVERDOSE?
Little is known about the effects of abacavir overdose but if an overdose occurs, treatment in a medical
center is needed. It is known in rats and mice that toxic levels of abacavir (7 to 24 times the normal level
for humans) cause heart degeneration over a period of two years. The clinical relevance of this finding
is unknown. If an overdose is suspected the local poison control center should be contacted or the
person should go to the nearest emergency room. There is no known antidote for abacavir if an
overdose occurs.
CAN ABACAVIR BE USED IN INFANTS?
Abacavir is not supposed to be used in children less than 3 months of age.
IS ABACAVIR EXCRETED IN BREAST MILK?
It is not known if abacavir is excreted in human breast milk but women with HIV should not breast feed
because of possibly transmitting HIV to an infant who is not infected. Abacavir is excreted in the breast
milk of lactating rats.
IS ABACAVIR SAFE TO USE DURING PREGNANCY?
It is unknown if abacavir is safe to use during pregnancy or if it harms an unborn child. Doctors
recommend that it only be used in pregnant women when the potential benefits outweigh the risks. HIV
medications are usually given to women with HIV because treatment is known to decrease the risk of
HIV transmission to the baby.
In a rabbit study, when abacavir was administered at 8.5 times the human exposure level, no birth
defects or developmental defects were noted. In rats who were administered abacavir at 35 times the
human exposure level, increased birth defects (e.g, skeletal malformations, generalized swelling) and
increased developmental abnormalities (e.g., decreased body weight and body length) occured. At half
of this dose, stillbirths and decreased body weight occurred.
IS THERE A GENERIC VERSION OF ABACAVIR AVAILABLE?
No.
HOW SHOULD ABACAVIR BE STORED?
Abacavir capsules and solution should be stored at room temperature (68 to 77 degrees Fahrenheit)
and away from excessive heat and moisture (e.g., not in the bathroom). The oral solution can be stored
at room temperature or be refrigerated but not frozen.
WHAT DOSE DOES ABACAVIR COME IN?
Abacavir is available in 300 mg tablets known as Ziagen. It is also available in a 20-mg/ml oral solution
(strawberry-banana flavored) also known as Ziagen. The oral solution is a clear to yellow color.
WHAT IS THE RECOMMENDED ABACAVIR DOSE?
The recommended abacavir dose is 300 mg twice a day or 600 mg once a day. This is the maximum
daily recommended dose. Dosage is based on one’s specific medical condition and response to
treatment. Children who are 3 months or older receive the oral solution of 8 mg/kg twice a day. Children
weighing more than 30.8 pounds can be treated with doses of 300 mg, 450 mg, or 600 mg based on
weight. There is a scored tablet available for children. If the child cannot swallow the tablet, then the oral
solution is used (240 ml per bottle). The bottle for the oral solution has a child resistant closure feature.
The doctor will determine the correct dose based on the child’s weight, not to exceed the recommended
adult dose. In general, children weighing between 30.8 and 46.3 pounds use 150 mg of abacavir in the
morning and evening for a total of 300 mg. Children weighing between 46.3 pounds 66.1 pounds usually
use 150 mg of abacavir in the morning and 300 mg at night for a total of 450 mg. Children weighing
more than 66.1 pounds usually use 300 mg of abacavir in the morning and 300 mg at night for a total of
600 mg.
Elderly people (age 65 or older) can use abacavir but it is unknown if they respond differently from
younger people. In general, abacavir is dosed cautiously in elderly people due to their greater frequency
of heart, liver, and kidney problems, other diseases, and use of other medications. What the body does
to the drug has not been studied in people age 65 or older. Research has showed no differences
between males and females or between blacks and Caucasians in what the body does to abacavir.
For patients with mild liver damage, doctors recommend that patients use 200 mg of abacavir twice a
day, which is 10 ml of the oral solution twice a day. Abacavir is not supposed to be used in patients with
moderate to severe liver damage because the safety, effectiveness, and how the body uses the
medication have not been established in such patients.
Doctors recommend that abacavir be taken about the same time each day because the medication
works best when it is kept in the body at a near constant level. It should continue to be used until the
doctor says otherwise, even if feeling well.
WHAT OTHER INGREDIENTS ARE IN ABACAVIR?
In addition to abacavir sulfate (active ingredient), the tablet also contains several inactive ingredients.
These ingredients are colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, and
sodium starch glycolate. Colloidal silicon dioxide is a substance used in tablet making to prevent caking
(lump formation), to create a film covering, to allow tablets to disintegrate, and to allow powder to flow
freely when tablets are processed. Magnesium stearate is a white powder that acts as a bulking agent
in tablet making. Microcrystalline cellulose is a term for refined wood pulp which is used to affect the
texture of tablets and to prevent caking. Sodium starch glycolate is a type of salt that helps tablets
rapidly disintegrate in the body and serves as a bulking agent.
The tablets have a film coating made of hypromellose, polysorbate 80, synthetic yellow iron oxide,
titanium dioxide, and triacetin. Hypromellose is a chemical that helps hold tablet ingredients together
and helps delay the release of medicine in the digestive tract. Polysorbate 80 is a type of bulking agent.
Synthetic yellow iron oxide is an artificial food coloring. Titanium dioxide is a powder made of fine
titanium bits. Triacetin is a type of fat.
The oral solution form of Abacavir contains the following inactive ingredients: artificial strawberry and
banana flavors, methylparaben and propylparaben (types of preservatives), citric acid as an acidic
flavoring agent, water, sorbitol (a type of sugar) solution, propylene glycol, saccharin sodium (a type of
sweetener), and sodium citrate. Propylene glycol is a substance that helps maintain water in
medications. Sodium citrate is a substance that helps alter and control acid levels.
DOES ABACAVIR NEED TO BE TAKEN WITH FOOD?
No, abacavir does not need to be taken with food because food does not affect how it is absorbed.
HOW DOES ABACAVIR INTERACT WITH ALCOHOL?
Abacavir does not interfere with the elimination of alcohol, at least in males. However, alcohol interferes
with the elimination of abacavir from the body in males. This can lead to increased levels of abacavir in
the body, which can increase side effects. This knowledge is based on research combining 600 mg of
abacavir with 5 alcoholic drinks in males, showing a 26% increase of abacavir in the body. Interactions
between alcohol and abacavir have not been studied in females.
DOES ABACAVIR IMPAIR FERTILITY?
Abacavir did not impair fertility based on a rat study in which rats were administered the medication at
about 8 times the human exposure level. It is not known if these results generalize to humans.
DOES ABACAVIR CAUSE CANCER?
Abacavir has been associated with an increased rates of cancer in rats who were administered the
medication over two years at three different dosage levels. However, the doses were 6 to 32 times the
human exposure recommended dose. Some of the cancer types were malignant (likely to spread and
invade other tissues) whereas other types were not malignant (benign). Malignant cancer was located
in male and female reproductive areas and in the liver of female rats. Benign cancer was located in the
liver and thyroid gland of female rats. The thyroid gland is a butterfly-shaped organ located in front of
the neck that produces a natural chemical known as hormones that affect virtually every cell in the body
and many functions such as disease fighting, heart rate, energy level, and skin condition.
DOES ABACACVIR CAUSE MUTATIONS?
Abacavir has caused mutations in chromosomes in a study of human lymphocytes. Chromosomes are
microscopic structures in cells that transmit genetic information. Abacavir has caused structural
changes in bone marrow for male mice but not female mice.
HOW IS ABACAVIR METABOLIZED?
Abacavir is partly metabolized (chemically transformed) by the alcohol-dehydrogenase enzyme system
in the liver, which is mainly responsible for breaking down alcohol. It is also metabolized by an enzyme
known as glucuronosyltransferase. The substances that result when abacavir is metabolized (which are
called metabolites) are known as 5'-carboxylic acid and 5'-glucuronide. These metabolites do not help
in fighting HIV.
WHAT IS THE HALF-LIFE OF ABACAVIR?
The half-life of abacavir is 1.5 hours. The half-life is the time that it takes the drug to fall to half of its
original amount in the body. 1.2% of abacavir is excreted in the urine as abacavir, 30% is excreted in
the urine as the metabolite as 5'-carboxylic acid, 36% is excreted in the urine as 5'-glucuronide, 15% is
excreted as unidentified metabolites in the urine, and 16% is eliminated in the feces. The half-life of
abacavir is known to be increased in patients with mild liver impairment. The rate by which abacavir
metabolites were formed and eliminated was decreased in patients with mild liver impairment.
WHAT ARE SOME OTHER CHEMICAL PROPERTIES OF ABACAVIR?
Abacavir has a plasma protein binding of 50%. Plasma protein binding reflects the degree to which a
drug binds to proteins in the blood. The less bound a drug is, the more it can exert its effect on the
body. 83% of the drug is available in the body’s circulation when administered by non-intravenous
routes. Overall, abacavir is rapidly and extensively absorbed by the body after oral administration. It
distributes easily into red blood cells. Red blood cells (RBCs) are cells that circulate in the blood that
specialize in delivering oxygen to the body’s tissues.
WHEN WAS ABACAVIR APPROVED AND WHEN DID THE PATENT EXPIRE?
Abacavir was approved by the FDA on 12/18/98 as the fifteenth retroviral drug (type of medication that
fights viruses) in the U.S. The patent on the drug expired on 12/26/09. Some laboratory versions of HIV
are resistant to Abacavir.
"Where Medical Information is Easy to Understand"™

Abacavir